The FDA twice declined to approve long-acting exenatide (Bydureon) in 2010, with its most serious concern being that the drug might contribute to heart rhythm abnormalities. There are safety concerns involving thyroid cancer and pancreatitis.
Bydureon is a longer-lasting version of Amylin’s existing drug Byetta, which is injected twice a day. Another company, Alkermes, supplied the technology that slowly releases Bydureon inside the body.
Bydureon, Byetta and Victoza are drugs called GLP-1 receptor agonists, which mimic the effect of glucagonlike peptide- 1, a hormone that increases insulin production when blood sugar is high.
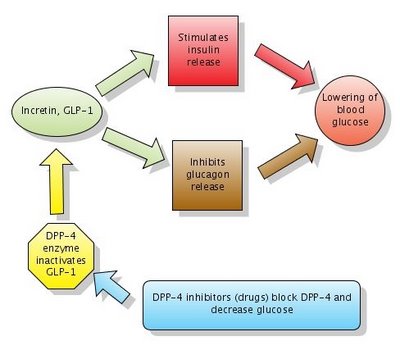
Figure: Action of DPP-4 inhibitors. Note that DPP-4 normally inactivates GLP-1. DPP-4 inhibitors block DPP-4 which in turn leaves GLP-1 active. Click to enlarge the figure. I made the figure with Gliffy in 2006 . The diagram Action of DPP-4 inhibitors is now widely used in many articles on Wikipedia, with my permission.
The main ingredient in both Bydureon and Byetta is exenatide, a hormone derived from the saliva of the Gila monster, a poisonous lizard found in the Southwestern United States and Mexico.
Wholesale price of Bydureon would be $323 for 4 doses, or about $4,200 a year. That is between the roughly $3,400 for the low dose of Victoza and $5,000 for the high dose.
References:
Diabetes Drug Injected Weekly Wins F.D.A. Approval. NYTimes.
Comments from Twitter:
Vaughn Eyvazian @Vaughnsays: Increase that pt compliance!
Reinaldo B. @basanezrx: but nobody stays in the lowest dose of Victoza in my experience, and that's why most ins companies pay for Byetta instead. A shame :(
Hiç yorum yok:
Yorum Gönder